The term ‘fossil fuels’ is commonly used for the materials tar sands, oil, gas condensates, natural gas, coal, and oil shale and their products. Petroleum comprises oil, gas condensate, and natural gas.
‘Petroleum’ is a term for a series of subsurface fluids, composed significantly, but not entirely, of hydrocarbons, which occur concentrated in reservoirs and can be produced at the surface as oil or gas, or both, and sometimes as liquid condensates. The term ‘hydrocarbon’ in its strict chemical sense refers to a chemical compound composed solely of hydrogen and carbon. Examples would be methane (CH4), a gas; decane (C10H22), an alkane and a liquid at room temperature and pressure; or phenanthrene (C14H10), an aromatic hydrocarbon and a solid at room temperature and pressure. All three occur naturally in solution in oil, and gas condensates; methane is the major component of most natural gases.
Petroleum also contains compounds other than hydrocarbons, including organic carbon compounds that contain oxygen, nitrogen, and especially sulphur. Normally it also contains significant quantities of non-hydrocarbon gases such as carbon dioxide, nitrogen, hydrogen sulphide, and traces of inert gases such as helium. The three commonly described forms of petroleum are natural gas, which does not condense at standard temperature and pressure; gas condensate, which is a gas-phase petroleum in the reservoir but partly condenses to a liquid (the condensate) at the surface; and oil, a petroleum that was liquid in the reservoir but produces a gas (solution gas) and a liquid phase, crude oil, at the surface. Some oils, such as those in tar sands, or very waxy oils from South-east Asia, may be solid or nearly solid at the surface.
Although usually dominated by the hydrocarbons, petroleums may contain up to 80 per cent or more of non-hydrocarbons. Most North Sea oils contain 50–90 per cent of hydrocarbons. Inorganic gases such as carbon dioxide, nitrogen, helium, and hydrogen sulphide are common components of petroleums, ranging in abundance from trace components to major fractions of the fluid.
Composition
Petroleum can be classified by its composition and the relative amounts of various chemical components.
Natural gases contain principally hydrocarbons with from 1–5 carbon atoms. They can exist in the reservoir as a gas phase or in solution in a liquid petroleum. In the reservoir under pressure, crude oil is a mixture of liquid hydrocarbons and non-hydrocarbons. It will contain many thousands of individual components with from 1 to 40 or more carbon atoms per molecule, although compounds with up to 100 or more carbon atoms are found. The largest molecules abundantly present in solution in crude oil will be the asphaltenes, which probably have up to an average of 70 or more carbon atoms, attendant hydrogen atoms, and several oxygen, nitrogen, or sulphur species per molecule. Some asphaltenes may have even larger numbers of carbon and associated atoms in their molecules. Asphaltenes represent small portions of source-rock organic matter dissolved in the crude oil medium. The average size of the molecules in a light crude oil would be about 15–20 carbon atoms per molecule. In most petroleums, even oils, methane is commonly the most abundant individual molecule. When oil is produced, the gas comes out of solution, leaving molecules with from 6 or more carbon atoms (the larger molecules) in the liquid oil.
Petroleums have a wide range of compositions but can be classified into three major types according to the relative proportions of gaseous (C1–C5) hydrocarbons and components with more than six carbon atoms (C6+). Natural gases are petroleums which are in the gas phase in the subsurface and which are composed of hydrocarbon mixtures dominated by methane with contributions from ethane, propane, butanes, and the pentanes and traces of inorganic gases, principally carbon dioxide and nitrogen. Gases by definition contain no C6+ components.
Gas condensates are petroleums that are the gas phase in the subsurface but contain significant amounts of C6+ components (condensate) in gaseous solution. These components include hydrocarbons and non-hydrocarbons, with up to more than 40 carbon atoms. The ratio (by mass) of gas (C1–C5) to C6+ components in gas condensates ranges from 3:1 up to very large values. On coming to the surface, as pressure and temperature are reduced in the petroleum production system, gas condensates undergo retrograde condensation, and a liquid phase (the condensate) condenses from the gas phase.
Oils are petroleums that are in the liquid phase in the subsurface but contain up to 50 weight per cent of gas in solution. According to the relative amount of solution gas, the dissolved gas, the oil is termed ‘black oil’ or ‘volatile oil’. This solution gas exsolves from the liquid during the reduction in pressure and temperature accompanying petroleum production. Oils and natural gases can coexist in contact in the same reservoir. In this case the oil is saturated with gas under reservoir conditions. Where no separate gas phase is present the oil may not be saturated with gas and is termed ‘undersaturated’.
The C6+ components of oils and condensates from the North Sea petroleum province are typically dominated by saturated hydrocarbons, with aromatic hydrocarbons in abundance; non-hydrocarbons (resins and asphaltenes) usually represent less than 30 per cent of the fluid. Other petroleums contain different proportions of these fractions, but most petroleums are dominated by the hydrocarbon fractions.
Petroleum systems
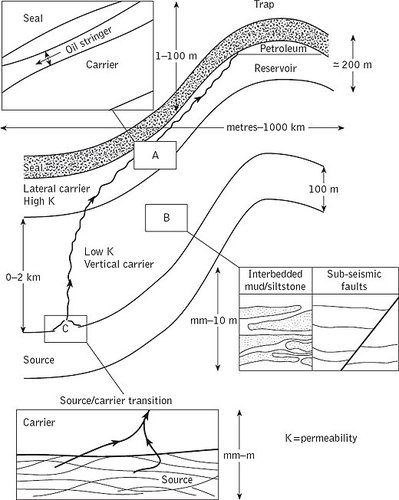
The elements of a petroleum system include the source rocks, the carrier beds, the trap, the reservoir and the seal (Fig. 1). The various processes acting on a petroleum system to produce an accumulation involve generation of petroleum in the source rocks, migration of petroleum from the source beds through the carrier beds and trapping of petroleum in the trap.
Fig. 1. A petroleum system consists of a source rock containing organic matter (kerogen), vertical and lateral carrier beds, a trap consisting of a reservoir rock, and a seal. Petroleum systems are usually less than 5 km deep; they can be very small laterally, with short migration distances, or range up to very large systems with lateral dimensions up to several hundred kilometres in foreland basins. Source rocks and reservoir rocks are typically about 100 metres thick. Vertical migration distances to a lateral carrier bed may be from 0 to several kilometres, and lateral migration distances can range up to several hundred kilometres. Vertical migration through mudstone carriers may take place through mudstone and interbedded siltstone pores, or through fracture systems.
Fig. 1. A petroleum system consists of a source rock containing organic matter (kerogen), vertical and lateral carrier beds, a trap consisting of a reservoir rock, and a seal. Petroleum systems are usually less than 5 km deep; they can be very small laterally, with short migration distances, or range up to very large systems with lateral dimensions up to several hundred kilometres in foreland basins. Source rocks and reservoir rocks are typically about 100 metres thick. Vertical migration distances to a lateral carrier bed may be from 0 to several kilometres, and lateral migration distances can range up to several hundred kilometres. Vertical migration through mudstone carriers may take place through mudstone and interbedded siltstone pores, or through fracture systems.
Source rocks and petroleum generation
Source rocks for oil and gas are usually fine-grained sediments, such as mudstones or marls rich in organic matter derived from bacterial and chemical alteration of algae, bacteria, or land-plant debris. Organic sediments, such as coals, can also act as source rocks, principally for natural gas and gas condensate. Where the coals are rich in hydrogen-rich land-plant debris, such as plant exines and resins, coals can also generate liquid petroleums, as is the case in parts of Australia. Source rocks are the site of petroleum generation from insoluble organic matter known as kerogen. Kerogen is the term used for the fractions of organic matter in a sediment that are insoluble in common organic solvents. It is the most abundant form of organic matter in the Earth's crust and is the chief organic component of oil shales, coals, source rocks generating crude oil and natural gas, and other sediments. It represents more, often much more, than 80 per cent by weight of the organic matter in these materials. Biochemical and chemical reactions convert reactive biochemical compounds, such as lipids, proteins, carbohydrates, and lignins, derived from dead organisms, to kerogen, biochemically unreactive, insoluble material, in shallow unconsolidated sediments. Some soluble organic matter is also present in all sediments, including coals, oil shales, source rocks, and other sediments and contributes in a minor way to any petroleum expelled from the source rock; but most petroleum is generated from alteration of the kerogen. As the source rock subsides in the sedimentary basin, it is heated to temperatures that are typically above 100 °C (Fig. 2). The complex kerogen decomposes thermally to produce oil and gas, which may migrate away from the source rock to produce a petroleum accumulation.
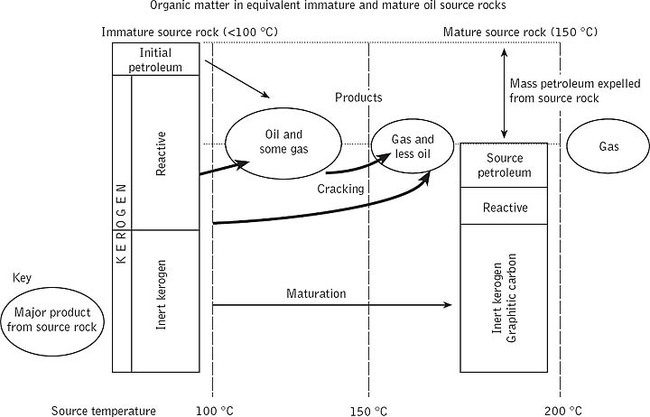
Fig. 2. Maturation converts immature organic matter in source rocks—principally kerogen—to oil and gas, and residual mature kerogen. The diagram shows the quantities of organic matter remaining in an immature source rock (temperature less than 100 °C) and a mature source rock (temperature: 150 °C). Both boxes are to the same scale, and the difference in heights represents the quantity of petroleum expelled from the source rock. At temperatures below 100 °C the kerogen is unaltered, but at higher temperatures up to 150 °C the reactive parts of the kerogen decompose to oil and associated solution gas and most of the oil and gas are expelled from a rich source rock. At temperatures over 150 °C any oil remaining in the source rock is converted to gas, which is expelled. Perhaps 50 per cent or more of the kerogen may generate petroleum in a good oil-generating source rock, and a large fraction of this would be expelled.
Fig. 2. Maturation converts immature organic matter in source rocks—principally kerogen—to oil and gas, and residual mature kerogen. The diagram shows the quantities of organic matter remaining in an immature source rock (temperature less than 100 °C) and a mature source rock (temperature: 150 °C). Both boxes are to the same scale, and the difference in heights represents the quantity of petroleum expelled from the source rock. At temperatures below 100 °C the kerogen is unaltered, but at higher temperatures up to 150 °C the reactive parts of the kerogen decompose to oil and associated solution gas and most of the oil and gas are expelled from a rich source rock. At temperatures over 150 °C any oil remaining in the source rock is converted to gas, which is expelled. Perhaps 50 per cent or more of the kerogen may generate petroleum in a good oil-generating source rock, and a large fraction of this would be expelled.
A source rock is thus a fine-grained sediment rich in organic matter that could generate (from kerogen) crude oil or natural gas on thermal alteration in the Earth's crust. The oil or gas could then migrate (move) away from the source rock to more porous and permeable sediments, where ultimately the oil or gas could accumulate to make a commercial accumulation. If a source rock has not been exposed to temperatures of about 100 °C in the Earth's crust, it is termed a potential source rock. If generation and expulsion of oil or gas have occurred, it is termed an actual source rock. The terms immature (not adequately heated) and mature (has been heated and generated crude oil and natural gas) are commonly used to describe source rocks.
Many of the major accumulations of oil on Earth were generated from organic-rich marine mudstones or marls with from 1 to 10 weight per cent organic carbon. Examples would include the Jurassic–Cretaceous source rocks of the Middle East fields, the Jurassic Kimmeridge Clay Formation of the North Sea basins, the Cretaceous La Luna Formation of Venezuela and Colombia, the Shublik, Kingak and Torok Formations of the Alaskan North Slope basins, the Miocene Monterey Formation of the California borderland basins, and the Devonian Duvernay, Jurassic Nordegg, and Cretaceous Speckled Shale Formations of the Western Canada sedimentary basin which have variously contributed to the vast Athabasca petroleum accumulation in western Canada. Very organic-rich shales or marls that have not been heated in the crust of the Earth may sometimes be termed oil shales.
Although most of the largest oilfields on Earth have originated from source rocks deposited in marine environments (marine shales), many major accumulations result from petroleum generation in non-marine sediments from, for example freshwater or saline organic-rich shales or coals deposited in lakes. Many of the accumulations of petroleum, both gas and oil, found in South-east Asia were generated in non-marine sediments. Examples of such source rocks gen-erating oil would be the Eocene Shahejie Formation shales of the Liaohe Basin in China. In all cases source rocks were deposited in slowly sedimenting basins with high surface biological productivity and restricted oxygenation of stagnant bottom waters resulting from bacterial consumption of free oxygen in bottom waters and sediments. Under such reduced oxygen conditions, large fractions, up to perhaps 1 to 10 per cent, of the primary biological productivity in the surface waters may have been preserved as kerogen and related bitumen-soluble components in sediments.
Petroleum generation
Most commercial source rocks contain 1–10 per cent by weight of organic carbon, mostly in the form of kerogen but with 5–15 per cent of the organic matter present being extractable. At temperatures above about 100 °C the kerogen decomposes and is converted to oil and/or gas depending on the type of organic matter in the sediments (typically over the temperature range 100–150 °C). Matter derived from algae and bacteria produces oil and associated gas. Matter derived from the woody parts of plants produces gas, principally at temperatures above, say, 150 °C. In the temperature range 100–150 °C, oil is generated in the crust and is expelled as a result of large pressure (fluid potential) gradients induced in the source rock as a result of the process of conversion of kerogen to oil and resulting compaction of the source rock. Generation and expulsion of oil from a source rock typically takes place at depths of about 3–5 km. Gas generation and expulsion continue at greater depths and at temperatures above about 150–160 °C.
In the thermal alteration of kerogen to oil and natural gas the solid-phase kerogen of high molecular weight becomes broken down to fluid products, such as oil and gas, which may migrate. While the average molecular weight of crude oil may be around 200–400, the mobile phase can contain asphaltenes, which are an operationally defined fraction of crude oil or solvent-soluble organic matter; they consist of material of higher molecular weight similar in bulk composition to source-rock kerogens. Asphaltenes may represent ‘kerogen-like’ material that is soluble in the fluid phase and may migrate with them.
All kerogens will generate petroleum mixtures on heating in the crust, but A. Pepper of BP suggests that not all kerogens efficiently expel the generated material from the source rock. Thus, most coals generate liquid materials but do not expel them; they retain the material in the coal matrix, where on further burial it becomes ‘cracked’ to gas, which may be expelled to migrate to a trap. Although all types of kerogen generate some petroleum on exposure to heat in the crust at depths below, say, 3 km (temperatures over 100 °C), not all types of material generate the same amount. Kerogens derived from algae can generate 40–80 per cent, by weight of petroleum components on maturation, whereas kerogens derived from higher plants generate 5–30 per cent, but usually less than 20 per cent by weight of petroleum (Fig. 2, reactive kerogen fraction).
Sediments rich in algal-derived kerogens (i.e. with total organic carbon contents of more than say 3 per cent by weight of the shale) can expel a large fraction of the generated crude oil and natural gas: as much as 80 per cent or more from some shales. As the richness of the sediment declines, the amount of crude oil and natural gas generated decreases and the proportion expelled also decreases. Thus source rocks lean in organic matter tend to be poor expellers of oils. Sediments in which only a small fraction of the kerogen is convertible to oil and natural gas are the poorest expellers of fluid-phase petroleums, and it is generally considered that coals rich in land-plant material are the least effective at expelling oils and typically expel only gas.
‘Coal’ and ‘oil shale’ are economic terms for organic-rich sediments. These may also be capable of generation of petroleum in sedimentary basins. In most instances coals principally generate and expel gas on heating in the subsurface. However, some coals derived from algae or from hydrogen-rich parts of plants such as cuticles and spores may generate and expel oil. These types of coals are common in South-east Asia and Australasia.
Although coals can generate the components present in oil and gas, the coal kerogen (principally in the maceral vitrinite) is rich in aromatic structures and is an efficient adsorber of all hydrocarbon and heterocompound components, but especially those components with molecules larger than methane. Most coals thus typically expel only gas. Coal kerogens contain many of the same basic chemical structural elements as algal kerogens, but coals usually have lower hydrogen contents than algal source rocks and generate smaller amounts of petroleum. This combination of a highly adsorbent aromatic base structure, together with a low yield of liquid product on generation typically results in only gas being expelled.
On a worldwide basis, the primary commercial source rocks for crude oil are marine or lacustrine shales rich in algal or bacterial organic matter (Alaska, the Middle East, Venezuela, Colombia, China, the North Sea, the Gulf of Mexico). D. McGregor of BP suggests that coal-bearing sequences are significant oil generators only in very specific and relatively uncommon geological settings. Coals and associated carbonaceous shales are thought to be the primary oil-prone source facies in Australia and an important secondary source in South-east Asia. Australian coals and associated carbonaceous sediments may thus well be oil source rocks in the Gippsland basin area and elsewhere in Australia. In other parts of the world there is no evidence that coals and carbonaceous shales have expelled major quantities of oil.
‘Oil shale’ is a term for shales that are very rich in organic material (see oil shale). They are essentially an organic rich immature source rock which has not been heated to a high enough temperature in the Earth's crust to convert the kerogen into crude oil and natural gas. There are no differences in the nature of the organic matter in crude oil source rocks and in oil shales except that oil shales would typically be very rich in organic matter and would have been prolific crude oil source rocks if they had been heated to temperatures of more than 100 °C in the Earth's crust (matured).
There is a wide variety of source rock types, and their behaviour on maturation varies with the composition of the organic matter, the organic content, and (to a lesser extent) the manner in which the organic matter and associated mineral content of the source rock are arranged. Two extreme end-member types of source rocks can be recognized.
Sediments such as shales rich in organic matter of algal and bacterial origin (1–10 per cent organic carbon by weight) start to generate oil and associated gas at temperatures about 100°C under normal geological sedimentary basin heating rates of 1–10 °C/My. At about 150–160 °C most of the potential of the kerogen to generate liquid hydrocarbon is exhausted, and above this temperature any oil remaining in the source rock will start to be thermally ‘cracked’ to shorter-chain compounds and gas. Above 200 °C little further potential for generation exists. With typical geothermal gradients of, say, 33 °C/km, oil generation (and expulsion) starts at about 3 km and peak amounts of oil are generated by 4.5 km. With a typical algal-derived kerogen, upwards of 50 per cent of the kerogen may be converted to oil in this depth/temperature range over a period of from 5 to 50 Ma with a normal heating rate during burial of the source rock. Such source rocks are typified by the upper Jurassic Kimmeridge Clay Formation of the North Sea basins.
Source rocks such as humic coals with a large input of land plant lignin probably generate liquid like petroleum, but because of the high sorbtion capacity of the coal kerogen for bitumen this is rarely expelled from the source rock. At temperatures above about 160 °C humic coal kerogen and any liquid petroleum trapped in the sediment becomes ‘cracked’ to natural gas or gas condensate, which is expelled. Such source rocks are typifled by the Carboniferous coals of western Europe. These have been the source of many major gas fields, including those in the southern North Sea basins, Holland, and Germany.
Migration of petroleum
‘Petroleum migration’ is the term for the series of processes by which petroleum is transported from its site of generation, the source rock, to the trap. ‘Primary migration’ is the term for the movement of petroleum from within the source rock to an adjacent carrier bed, at distances typically up to a maximum of a few hundred metres. ‘Secondary migration’ is the term describing the movement of petroleum from the source rock–carrier bed contact to the trap. A ‘carrier bed’ is a porous and permeable rock near the source rock through which petroleum flows from source to trap. Carrier-bed rocks are lithologically the same as reservoir rocks, that is, sandstones, limestones, or fractured rocks of all types. In some instances fluid-phase oil and natural gas may migrate through fine-grained rocks such as mudstones. Such is the case in the Gulf of Mexico, where petroleum migrates vertically over several kilometres vertically through mudstones and faults assisted by very high pressures in the deep sediments.
The principal driving force that moves the fluid-phase, crude oil and natural gas, from source to trap in most permeable carrier beds is buoyancy; the fluid-phase crude oil and natural gas in the deep subsurface have densities in the region of 500–800 kg m−3, whereas waters in sediments have densities of over 1000 kg m−3. The oil flows through the pore systems of the carrier beds as rivers of high oil saturation; most of the carrier system does not contain any oil. Even where the reservoir is a fractured source rock, some internal flow (migration) of oil is involved in concentrating the oil to form an accumulation.
The distance from the petroleum source rock to the trap may be as large as many hundreds of kilometres or very short, as where petroleum is trapped in high-permeability fractures within the source rock itself, as in the Bakken Formation oilfields of North Dakota. In the North Sea petroleum basins secondary migration distances are typically of the order of 20–40 km from source to trap, but fields such as the giant Troll field in the Norwegian sector may drain petroleum from source rocks over 100 km from the trap. The rates at which oil and gas move in the subsurface during secondary migration can be very high. Oyvind Sylte at the Continental Shelf Institute in Norway suggests that in the North Sea once generated, petroleum may in some instances flow through the carrier from source to trap in as little as 10 000–100 000 years. The distances can also be impressive: J. Allan and S. Creaney of Esso consider that petroleum from Cretaceous or Devonian source rocks may have migrated over 400–500 km laterally from the source rocks in the Western Canada basin.
The primary migration of petroleum from source rocks is still the subject of much debate, but some understanding of the process has been achieved for the most common types of source rocks: those rich shale source rocks that generate most of the world's oil. Migration of crude oil and natural gas from oil-prone algal source rocks proceeds in a complex manner but is probably related to compaction of the source rock. As the solid kerogen phase converts to a fluid phase (solvent-soluble organic matter), the fluid phase has to bear part of the load of the overlying sediments that was previously borne by the solid kerogen. In consequence, fluid pressures in the source rock rise and the fluid phase is driven out of the source rock through fractures induced during the overpressuring, through the pore system of the source rock, and also partly in solution in the remaining kerogen matrix. Once in the carrier bed (outside the source rock) it is buoyancy that principally causes the oil to flow from source to reservoir; the oil being less dense than the surrounding waters in the rock-pore systems. In most oil source rocks the gas associated with the oil is expelled in solution in the oil.
In secondary migration in the carrier bed, petroleum moves updip in a carrier rock predominantly because of its buoyancy, since it has a lower density than the surrounding formation waters. Petroleums in the subsurface will typically have densities ranging from 800 to 500 kg m−3 for oils and as low as 100 kg m−3 for gases according to the pressure and temperature. In contrast, formation waters typically have subsurface densities greater than 1000 kg m−3. Regional hydrodynamic gradients driving water flow in the basin may have a very minor role in petroleum migration, but petroleum is considered to move as a separate phase from the waters in basins. In many basins there is in fact little or no water flow associated with petroleum migration.
Theoretical studies by W. England of BP and examination of migration pathways in the field clearly indicate that petroleum does not flow through the entire carrier bed but tends to move in concentrated zones where the petroleum has displaced most of the formation water. Petroleum flow perhaps takes place in as little as 1–10 per cent of the total potential pore space of a carrier bed, the petroleum moving in zones in which it occupies most of the pore space. These petroleum rivers probably have lateral and vertical dimensions a few metres to tens of metres in extent; away from these zones most of the carrier system pore space probably contains only water.
Reservoir rocks and traps
If petroleum is to be produced from the subsurface it must accumulate in rocks that are porous and permeable enough for reasonable volumes of petroleum to be produced at reasonable cost. Most reservoir rocks have permeabilities greater than 1 millidarcy; some have permeabilities of many darcies (1 darcy is 10−12 m2). Most reservoir rocks are sediments such as sandstones, conglomerates, and limestones with porosities typically ranging from 10 to 30 per cent, the petroleum being contained in the pores of the sediments. Petroleum can also accumulate in fractures in limestones, or even within the source rocks themselves. Examples of reservoir rocks include the Middle Jurassic Brent Group sandstones or the Upper Cretaceous fractured Chalk of the North Sea basins, which contain both oil and gas. Reservoired petroleum columns may be as thick as several hundred metres. The petroleum typically fills 50–90 per cent of the pore space, the remainder of the pore space containing formation water trapped when the petroleum entered the field.
In order for petroleum to accumulate in a trap, several criteria must be satisfied. The crude oil and natural gas is trapped in a porous and permeable rock body: a reservoir rock encased above, and sometimes below, by fine-grained rocks that are relatively impermeable to crude oil—the seals. Crude oil (and gas) is kept in the trap by the buoyancy forces acting on it, which tend to move the oil to the surface of the sedimentary basin but are prevented from doing so by the seals. The trap must completely seal an area of reservoir rock; if it does not, no petroleum accumulation will develop. Most seals are fine-grained rocks such as shales or mudstones. The best seals are salt beds, which can even trap gas for long periods of geological time. Lateral sealing is accomplished either by structural deformation of the reservoir rock and seal into a closed anticline or by the reservoir rock changing laterally into a seal rock such as a shale. Faulting of the trap might also place a shale next to a reservoir rock and thus seal the trap.
The oil usually occupies about 50–80 per cent of the pores of the reservoir rock, the remainder of the pore volume being occupied by water. In some accumulations the reservoir can be filled by gas or both gas and oil may occupy the pore volume. Oil reservoirs can occur at any depth within the crust but liquid oil accumulations are rarely found deeper than 5 km, for the temperatures at greater depths may be so high (more than 160 °C) that any oil in the reservoir would be cracked to gas.
Petroleum accumulations are ephemeral features; the petroleum often leaks through the seals with the passage of time. It is thought that most of the petroleum generated during the history of the Earth has leaked away and has been destroyed at the surface by bacterial action and evaporation. Most of the world's existing large petroleum accumulations were generated and migrated within the past few tens of millions of years, and few petroleum accumulations have been found that have contained petroleum for more than 100 million years.
Petroleum alteration
Once in a trap, petroleum can have its composition altered by a variety of processes, which usually reduce the commercial value of the oil and complicate extraction of the petroleum. Five types of processes are involved in petroleum alteration in the reservoir: thermal degradation, biodegradation, thermochemical sulphate reduction, water washing, and asphaltene precipitation.
Thermal degradation or cracking affects liquid petroleum trapped in reservoirs hotter than about 160 °C. Heavier fractions of the petroleum are broken down to lighter fractions, including large amounts of methane and other gases. At temperatures over 200 °C even gases such as propane or butane may be broken down to methane. If degradation is severe a carbon-rich deposit, pyrobitumen, may form in the reservoir, blocking the pores and preventing easy production of the petroleum.
Bacterial action, or biodegradation, affects oils, and, to a lesser extent, natural gases in shallower petroleum accumulations where the reservoir temperature is less than about 70 °C, where there are nutrients such as phosphate ion, and where a chemical oxidant is present in the reservoir waters. Traditionally the oxidant is thought to be molecular oxygen brought into the reservoir by slowly moving groundwater, but this idea is increasingly being challenged and other oxidants are undoubtedly involved. Oxidation of the hydrocarbons in the petroleum results in the production of a heavier, more viscous oil with lower economic value that is more difficult to refine. Perhaps more than half the oil on Earth has been biodegraded. The largest accumulations of oil are the giant heavy oil accumulations of the Canadian and Venezuelan heavy oil belt. The Alberta heavy oil deposit alone contains over 2500 billion barrels of petroleum which can be produced by mining and extraction of the ‘heavy oil’. Although less familiar than the giant accumulations of oil in the middle East, these biodegraded oils will become more economically important in the twenty-first century when the conventional, easily produced, crude oils have been largely consumed.
Thermochemical sulphate reduction is a less common alteration process in which methane, and sometimes other hydrocarbons, in the petroleum react with sulphate ion in the formation waters to produce hydrogen sulphide and remove the hydrocarbon. This lowers the value of the deposit. This alteration process takes place only in reservoirs where the temperature is greater than 140 °C and abundant sulphate ion is present in the water. Gases rich in hydrogen sulphide associated with thermochemical sulphate reduction are common features of petroleums found in deep reservoirs in the Gulf of Mexico and Alberta.
Water washing results from the removal of water-soluble components in the petroleum by slowly moving groundwater in the reservoir. Water washing usually accompanies biodegradation and is commonly associated with shallow petroleum accumulations close to mountain ranges or other elevated terrain where groundwater at a high level can drive water flow in the subsurface.
Asphaltene precipitation results when gas, usually rich in methane, enters an oil reservoir and causes precipitation of the heaviest fractions of the oil. The asphaltenes, with about 70 carbon atoms per molecule, are the largest molecules dissolved in oil. They become insoluble when the oil becomes charged with much gas and they precipitate to form a feature known as a tar mat. Several large oilfields in the world have thick tar mats. The Prudhoe Bay field in Alaska and the Oseberg oilfield in the North Sea both have tar mats more than 10 metres thick at the base of the oil column in the trap. Tar mats complicate the production of the oil. When they are present, asphaltene deposits also form in the production piping, causing production problems and blockages.
The future
Petroleum is a finite natural resource that has been rapidly consumed by man in the twentieth century. As Hubbert showed, when world demand for petroleum rises, petroleum production must pass through a maximum, and eventually the remaining discoveries must be smaller and smaller irrespective of the additional exploration effort. Although gas reserves will last well into the twenty-first century, oil production rates in the USA peaked in the 1970s, and those in the North Sea in the late 1990s. Colin Campbell estimates that world production rates will peak in the decade 2000–10 at above 60–70 million barrels a day. From then on world petroleum production will decline to a level below about 20 million barrels a day. By 2050 other energy sources will be needed to replace the demand. With increased use of heavy oil and tar sands as the lighter lower-viscosity oils are consumed, it is likely that petroleum will form a small part of the energy supply until well into the second half of the twenty-first century. Total recovery of oil will amount to about 1800 billion barrels.
Bibliography and More Information about petroleum
- Hunt, J. M. (1979) Petroleum geochemistry and geology. W. H. Freeman, New York.
- Tissot, B. P. and Welte, D. H. (1984) Petroleum formation and occurrence. Springer-Verlag, Berlin.


Tidak ada komentar:
Posting Komentar